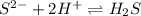Chemistry, 21.08.2019 05:30, emmareese2022
The best explanation for the dissolution of zns in dilute hcl is that a. the sulfide ion concentration is decreased by the formation of h2s. b. the zinc ion is amphoteric. c. the zinc ion concentration is decreased by the formation of a chloro complex. d. the sulfide ion concentration is decreased by oxidation to sulfur. e. the solubility product of zncl2 is less than that of zns.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, hsjsjsjdjjd
In which of the following belong to a category called the main group of elements? nonmetals. transition elements. halogens. alkali metals. it can be more than one answer!
Answers: 3


Do you know the correct answer?
The best explanation for the dissolution of zns in dilute hcl is that a. the sulfide ion concentrati...
Questions in other subjects:





Mathematics, 01.09.2019 04:30



Biology, 01.09.2019 04:30

Mathematics, 01.09.2019 04:30


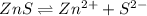
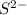 is a strong conjugate base of weak acid
is a strong conjugate base of weak acid 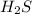 .
.