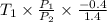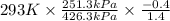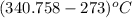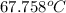Chemistry, 20.08.2019 23:30, kailibug2287
Aclosed tank contains oxygen at 20°c at a gage pressure of 150 kpa. determine the temperature if the oxygen is compressed isentropically to a gage pressure of 325 kpa. the atmospheric pressure is 101.3 kpa and the specific heat ratio of oxygen is 1.40. express your answer in °c to three significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Do you know the correct answer?
Aclosed tank contains oxygen at 20°c at a gage pressure of 150 kpa. determine the temperature if the...
Questions in other subjects:

Mathematics, 21.04.2020 05:55


Social Studies, 21.04.2020 05:55

English, 21.04.2020 05:55

Mathematics, 21.04.2020 05:55

Social Studies, 21.04.2020 05:55





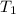 =
= 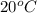 = (20 + 273) K = 293 K
= (20 + 273) K = 293 K 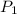 ) will be (150 kPa + 101.3 kPa) = 251.3 kPa
) will be (150 kPa + 101.3 kPa) = 251.3 kPa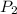 ) will be (325 kPa + 101.3 kPa) = 426.3 kPa
) will be (325 kPa + 101.3 kPa) = 426.3 kPa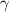 )is given as 1.40.
)is given as 1.40.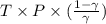 = constant
= constant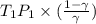 =
= 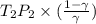
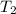 =
=