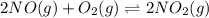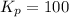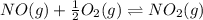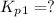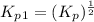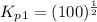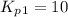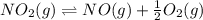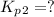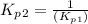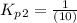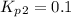Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 02:00, Robloxdemonduckyt
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

Chemistry, 23.06.2019 05:00, pmbeachy3102
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
Do you know the correct answer?
Find the equilibrium constants, kp, for the following equilibria, (i) no(g) + ½ o2(g) ⇄ no2(g), kp =...
Questions in other subjects:

Social Studies, 23.05.2021 01:00

Geography, 23.05.2021 01:00


Mathematics, 23.05.2021 01:00

Mathematics, 23.05.2021 01:00



Mathematics, 23.05.2021 01:00

English, 23.05.2021 01:00