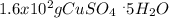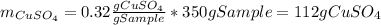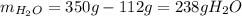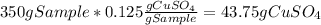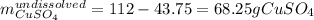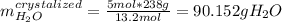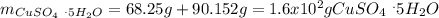Asaturated solution prepared at 70∘c contains 32.0 g cuso4 per 100.0 g solution. a 350 −g sample of this solution is then cooled to 0∘c and cuso4⋅5h2o crystallizes out. if the concentration of a saturated solution at 0∘c is 12.5 gcuso4/100 g soln, what mass of cuso4⋅5h2o would be obtained? [hint: note that the solution composition is stated in terms of cuso4 and that the solid that crystallizes is the hydrate cuso4⋅5h2o.] express your answer using two significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
Do you know the correct answer?
Asaturated solution prepared at 70∘c contains 32.0 g cuso4 per 100.0 g solution. a 350 −g sample of...
Questions in other subjects:

Mathematics, 01.03.2020 19:00

Mathematics, 01.03.2020 19:00


Mathematics, 01.03.2020 19:02

Mathematics, 01.03.2020 19:11

English, 01.03.2020 19:11

Chemistry, 01.03.2020 19:12

Mathematics, 01.03.2020 19:12

Biology, 01.03.2020 19:12