
Chemistry, 20.08.2019 22:20, davionb556
Consider the following reaction where kc = 1.80×10-2 at 698 k: 2 hi (g) h2 (g) + i2 (g)a reaction mixture was found to contain 0.304 moles of hi (g), 5.07×10-2 moles of h2 (g), and 4.57×10-2 moles of i2 (g), in a 1.00 liter container. indicate true (t) or false (f) for each of the following: 1. in order to reach equilibrium hi(g) must be produced .2. in order to reach equilibrium kc must increase .3. in order to reach equilibrium h2 must be consumed .4. qc is less than kc.5. the reaction is at equilibrium. no further reaction will occur.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 00:20, destromero
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
Do you know the correct answer?
Consider the following reaction where kc = 1.80×10-2 at 698 k: 2 hi (g) h2 (g) + i2 (g)a reaction mi...
Questions in other subjects:

Physics, 10.03.2020 22:28

Mathematics, 10.03.2020 22:28



English, 10.03.2020 22:28

Mathematics, 10.03.2020 22:28





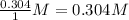
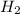 =
= 
 =
= 
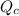 , for this reaction =
, for this reaction = ![\frac{[H_{2}][I_{2}]}{[HI]^{2}}](/tpl/images/0182/8823/7ae06.png)

 therefore reaction must run in reverse direction to reduce
therefore reaction must run in reverse direction to reduce 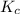 . That means HI(g) must be produced and
. That means HI(g) must be produced and 



