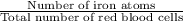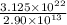Chemistry, 20.08.2019 05:20, aliveajones2005
Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body. in the blood of an adult human, there are approximately 2.64 × 1013 red blood cells with a total of 2.90 g of iron. on the average, how many iron atoms are present in each red blood cell? (molar mass fe = 55.85 g/mol)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2


Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Do you know the correct answer?
Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the v...
Questions in other subjects:




Mathematics, 08.07.2019 12:30

Mathematics, 08.07.2019 12:30

Mathematics, 08.07.2019 12:30

Biology, 08.07.2019 12:30



World Languages, 08.07.2019 12:30


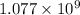
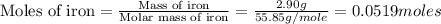
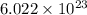 number of iron atoms
number of iron atoms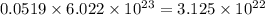 number of iron atoms
number of iron atoms