
Chemistry, 20.08.2019 02:30, notseansafe
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium constant for this reaction will
a. increase if the temperature is decreased.
b. decrease with increasing temperature.
c. increase with increasing temperature.
d. increase at some pressures and decrease at other pressures.
e. not change if the temperature is increased.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2


Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
Do you know the correct answer?
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium con...
Questions in other subjects:

Mathematics, 08.04.2021 21:30



Mathematics, 08.04.2021 21:30



Mathematics, 08.04.2021 21:30




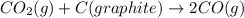
![K_{c} = \frac{[CO_{2}]}{[CO]^{2}}](/tpl/images/0180/4423/2161b.png)




