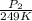The vapor pressure of liquid iodomethane, ch3i, is 40.0 mm hg at 249 k. a sample of ch3i is placed in a closed, evacuated container of constant volume at a temperature of 404 k. it is found that all of the ch3i is in the vapor phase and that the pressure is 72.0 mm hg. if the temperature in the container is reduced to 249 k, which of the following statements are correct
choose all that apply
a. the pressure in the container will be 104 mm hg.
b. no condensation will occur.
c. some of the vapor initially present will condense.
d. only pentane vapor will be present.
e. liquid pentane will be present.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 22:00, luciaaviles3
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
Do you know the correct answer?
The vapor pressure of liquid iodomethane, ch3i, is 40.0 mm hg at 249 k. a sample of ch3i is placed i...
Questions in other subjects:







Chemistry, 19.07.2019 09:20


Biology, 19.07.2019 09:20

Biology, 19.07.2019 09:20


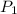 = 72.0 mm Hg,
= 72.0 mm Hg, 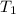 = 404 K
= 404 K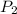 = ? ,
= ? , 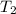 = 249 K
= 249 K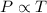 (at constant volume)
(at constant volume)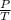 = k
= k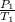 =
= 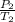
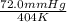 =
=