

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 21.06.2019 23:50, scavalieri2421
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
Do you know the correct answer?
Classify each of the following substances as either a strong or weak acid, strong or weak base, or a...
Questions in other subjects:





History, 12.10.2019 18:00



History, 12.10.2019 18:00



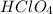 is a strong acid,
is a strong acid, 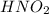 is a weak acid, NaBr is a soluble salt and
is a weak acid, NaBr is a soluble salt and 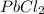 is an insoluble salt
is an insoluble salt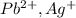 and Hg^{+}[/tex].
and Hg^{+}[/tex].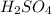 ,
, 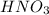 ,
, 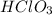 and
and 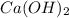 ,
, 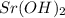 and
and 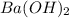 are strong bases.
are strong bases.



