
Chemistry, 18.08.2019 04:10, kadence428
The cell potential of the following electrochemical cell depends on the gold concentration in the cathode half-cell: pt(s)|h2(g,1atm)|h+(aq,1.0m)|au3+(a q,? m)|au(s). what is the concentration of au3+ in the solution if ecell is 1.27 v ? express your answer using two significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kaliloabousjbf
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
Do you know the correct answer?
The cell potential of the following electrochemical cell depends on the gold concentration in the ca...
Questions in other subjects:

Mathematics, 18.08.2020 09:01

Mathematics, 18.08.2020 09:01

Mathematics, 18.08.2020 09:01

Mathematics, 18.08.2020 09:01


Mathematics, 18.08.2020 09:01




Mathematics, 18.08.2020 09:01


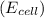 is given as 1.27 V.
is given as 1.27 V.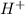 = 1.0 M
= 1.0 M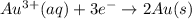
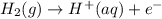
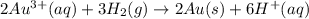
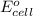 ) for hydrogen is equal to zero.
) for hydrogen is equal to zero.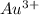 is
is  equal 1.50 V.
equal 1.50 V.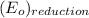 -
- 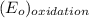
![E_{cell} = E^{o}_{cell} - \frac{0.059}{2}log \frac{[H^{+}]^{6}}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/b4490.png)
![log\frac{1}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/7ec17.png) = 23
= 23![\frac{1}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/cebd5.png) =
= 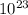
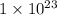
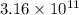
![[Au^{3+}]](/tpl/images/0175/5466/7b483.png) =
= 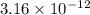 M
M



