
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate the molar mass of chloral hydrate. b. what amount (moles) of c2h3cl3o2 molecules are in 500.0 g chloral hydrate? c. what is the mass in grams of 2.0 x 10-2 mol chloral hydrate? d. what number of chlorine atoms are in 5.0 g chloral hydrate? e. what mass of chloral hydrate would contain 1.0 g cl? f. what is the mass of exactly 500 molecules of chloral hydrate?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
Do you know the correct answer?
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate...
a. calculate...
Questions in other subjects:



Mathematics, 13.09.2021 23:20

Biology, 13.09.2021 23:20

Mathematics, 13.09.2021 23:20

Mathematics, 13.09.2021 23:20

Chemistry, 13.09.2021 23:20



History, 13.09.2021 23:20


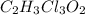 is, 165.5 g/mole
is, 165.5 g/mole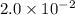 mole chloral hydrate is, 3.31 g
mole chloral hydrate is, 3.31 g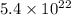

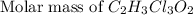 =2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]
=2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]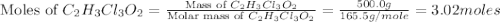
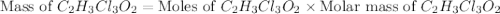
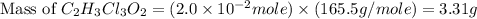
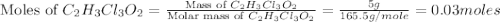
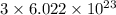 chlorine atoms
chlorine atoms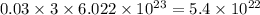 chlorine atoms
chlorine atoms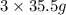 of chlorine present in 165.5 g of
of chlorine present in 165.5 g of 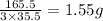 of
of 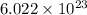 molecules of chloral hydrate has 165.5 g mass of chloral hydrate
molecules of chloral hydrate has 165.5 g mass of chloral hydrate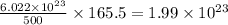 mass of chloral hydrate
mass of chloral hydrate



