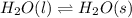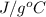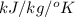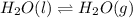Question 2: phase changes (14 points) a. rewrite each of the following equations for phase changes, to include the heat required for the phase change. use values from latent heat and specific heat constant tables when necessary. indicate whether each phase change is endothermic or exothermic. (2 points) i. h2o(l) h2o(s) (1 point) ii. h2o(l) h2o(g) (1 point)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, boonkgang6821
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1


Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
Do you know the correct answer?
Question 2: phase changes (14 points) a. rewrite each of the following equations for phase changes,...
Questions in other subjects:

Mathematics, 14.10.2020 16:01

Mathematics, 14.10.2020 16:01

French, 14.10.2020 16:01



Computers and Technology, 14.10.2020 16:01

Chemistry, 14.10.2020 16:01

Mathematics, 14.10.2020 16:01


English, 14.10.2020 16:01