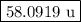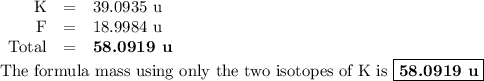Chemistry, 14.08.2019 08:20, petroale000
The two isotopes of potassium with significant abundance in nature are 39k asotopic mass 38.9637 amu, 932.58%) and 41k osotopic mass 40.9618 amu, 6.730%). fluorine has only one naturally occurring isotope, 19f (isotopic mass 18.9984 amu). calculate the formula mass of potassium fluoride.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3
Do you know the correct answer?
The two isotopes of potassium with significant abundance in nature are 39k asotopic mass 38.9637 amu,...
Questions in other subjects:




Mathematics, 17.04.2020 01:03


Mathematics, 17.04.2020 01:03

Mathematics, 17.04.2020 01:03