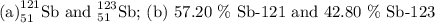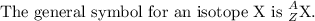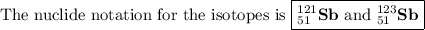Chemistry, 14.08.2019 08:20, sarahkuener
Antimony has many uses, for example, in infrared devices and as part of an alloy in lead storage batteries. the element has two naturally occurring isotopes, one with mass 120.904 amu. the other with mass 122904 amu. (a) write the azx notation for each isotope, (b) use the atomic mass of antimony from the periodic table to calculate the natural abundance of each isotope.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
Do you know the correct answer?
Antimony has many uses, for example, in infrared devices and as part of an alloy in lead storage bat...
Questions in other subjects:





English, 26.03.2020 20:09