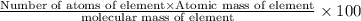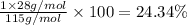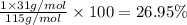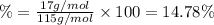Chemistry, 14.08.2019 08:10, anoyinpokep3c3sg
Ammonium dihydrogen phosphate, formed from the reaction of phosphoric acid with ammonia, is used as a crop fertilizer as well as a component of some fire extinguishers, (a) what are the mass percentages of n and p in the compound? (b) how much ammonia is incorporated into 100. g of compound?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, badgirl2005
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
Do you know the correct answer?
Ammonium dihydrogen phosphate, formed from the reaction of phosphoric acid with ammonia, is used as...
Questions in other subjects:

Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00



English, 03.02.2021 01:00

Computers and Technology, 03.02.2021 01:00


Social Studies, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00


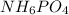 .
.