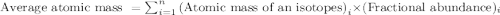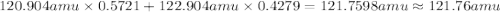Chemistry, 13.08.2019 06:10, QuestionsAnsweredNow
The two naturally occurring isotopes of antimony, 121sb (57.21 percent) and 123sb (42.79 percent), have masses of 120.904 and 122.904 amu, respectively. what is the average atomic mass of sb? (a) 121.90 amu (b) 122.05 amu (c) 121.76 amu (d) 121.34 amu (e) 122.18 amu

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kristineford198
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3


Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
Do you know the correct answer?
The two naturally occurring isotopes of antimony, 121sb (57.21 percent) and 123sb (42.79 percent), h...
Questions in other subjects:

English, 09.06.2021 21:00



Biology, 09.06.2021 21:00

Mathematics, 09.06.2021 21:00