
Chemistry, 13.08.2019 05:10, natajaeecarr
Below is a proposed mechanism for the decomposition of h2o2. h2o2 + i– → h2o + io– slow h2o2 + io– → h2o + o2 + i– fast which of the following statements is incorrect? a. io– is a catalyst. b. the reaction is first-order with respect to [i–]. c. the reaction is first-order with respect to [h2o2]. d. the net reaction is 2h2o2 → 2h2o + o2. e. i– is a catalyst.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 22:00, luciaaviles3
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
Do you know the correct answer?
Below is a proposed mechanism for the decomposition of h2o2. h2o2 + i– → h2o + io– slow h2o2 + io– →...
Questions in other subjects:

Mathematics, 03.05.2021 21:40


History, 03.05.2021 21:40


Mathematics, 03.05.2021 21:40

English, 03.05.2021 21:40

Mathematics, 03.05.2021 21:40




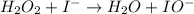 (slow)
(slow)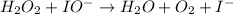
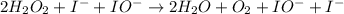
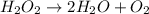
![k[H_{2}O_{2}][I^{-}]](/tpl/images/0174/7990/a32b4.png)
![[I^{-}]](/tpl/images/0174/7990/13772.png) and it is also first order reaction with respect to
and it is also first order reaction with respect to ![[H_{2}O_{2}]](/tpl/images/0174/7990/955b6.png) .
.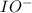 is a catalyst.
is a catalyst.



