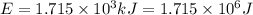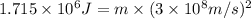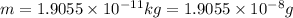In the second footnote it was pointed out that mass and energy are alternate aspects of a single entity called mass-energy. the relationship between these two physical quantities is einstein's equation, e= mc^2, where e is energy, m is mass, and c is the speed of light. in a combustion experiment, it was found that 12.096 g of hydrogen molecules combined with 96.000 g of oxygen molecules to form water and released 1.715 x 10^3 kj of heat. use einstein's equation to calculate the corresponding mass change in this process, and comment on whether or not the law of conservation of mass holds for ordinary chemical processes.

Answers: 3
Similar questions


Physics, 02.07.2019 00:00, ohshushan2
Answers: 1

Biology, 09.10.2019 17:30, janeou17xn
Answers: 1
Do you know the correct answer?
In the second footnote it was pointed out that mass and energy are alternate aspects of a single ent...
Questions in other subjects:

Mathematics, 22.07.2019 13:30

Mathematics, 22.07.2019 13:30





Health, 22.07.2019 13:30


Social Studies, 22.07.2019 13:30


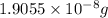
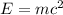 ,
,