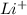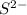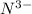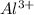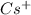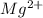Chemistry, 13.08.2019 04:30, alexandergonzalez38
Write the formula of the common ion derived from each of the following: (a) li, (b) s, (c) l, (d) n, (e) al, (f ) cs, (g) mg.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, Serenitybella
Drag each tile to the correct box arrange the layers not order from oldest to youngest
Answers: 2

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2
Do you know the correct answer?
Write the formula of the common ion derived from each of the following: (a) li, (b) s, (c) l, (d) n...
Questions in other subjects:

Chemistry, 23.07.2021 20:00



Mathematics, 23.07.2021 20:00


Mathematics, 23.07.2021 20:00

Mathematics, 23.07.2021 20:00

English, 23.07.2021 20:00


Computers and Technology, 23.07.2021 20:00