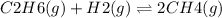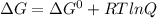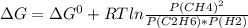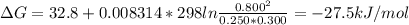For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 atm for all species. for the reaction c2h6(g)+h2(g)↽−−⇀2ch4(g) the standard change in gibbs free energy is δ°=−32.8 kj/mol . what is δg for this reaction at 298 k when the partial pressures are =0.250 atm , =0.300 atm , and =0.800 atm ?

Answers: 2
Similar questions

Chemistry, 02.07.2019 06:10, neverender098
Answers: 3

Chemistry, 21.08.2019 19:30, bnbjj
Answers: 3

Chemistry, 09.10.2019 23:00, mattosmiriah5533
Answers: 2
Do you know the correct answer?
For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 atm for all specie...
Questions in other subjects:


Advanced Placement (AP), 25.03.2021 20:10

Mathematics, 25.03.2021 20:10

English, 25.03.2021 20:10



Mathematics, 25.03.2021 20:10