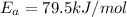Chemistry, 13.08.2019 02:20, dramaqueenactr2040
The rate constants for the first-order decomposition of a compound are 5.22× 10–4 s–1 at 43°c and 2.91 × 10–3 s–1 at 62°c. what is the value of the activation energy for this reaction? (r = 8.31 j/(mol · k)) a. 79.5 kj/mol b. 34.5 kj/mol c. 0.751 kj/mol d. 0.87104 kj/mol e. 2 kj/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 11:30, ghari112345
Which of the following is the most likeley example of an favorable mutation a. a mutation that makes a rabbit able run faster b. a mutation that changes the rabbit's fur to bright orange c. a mutation that changes the color of the rabbit's eyes d. a mutation that gives a rabbit a third ear
Answers: 1

Chemistry, 23.06.2019 12:40, valleriieZ7002
Metric temperature is measured in celsius and fahrenheit. true or false
Answers: 2
Do you know the correct answer?
The rate constants for the first-order decomposition of a compound are 5.22× 10–4 s–1 at 43°c and 2....
Questions in other subjects:

History, 01.08.2019 07:30




Mathematics, 01.08.2019 07:30

Mathematics, 01.08.2019 07:30

Arts, 01.08.2019 07:30


Mathematics, 01.08.2019 07:30



 is the activation energy and T is temperature in kelvin
is the activation energy and T is temperature in kelvin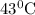 ,
,  ............(1)
............(1)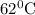 ,
, 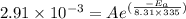 ............(2)
............(2)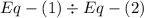 gives-
gives-