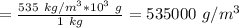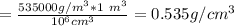Chemistry, 13.08.2019 00:30, Chandler1Gaming
The density of lithium metal is 535 kg/m3. what is this density in g/cm3? a) 0.000535 g/cm^3 b) 0.535 g/cm^3 c) 0.0535 g/cm^3 d) 0.54 g/cm^3 e) 53.5 g/cm^3

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 07:30, jessicawelch25
In a laboratory determination of the atomic weight of tin, a sample of tin is weighed in a crucible. nitric acid is added, and the reaction proceeds to give a hydrated tin(iv)oxide plus no2and h2o. the hydrated tin(iv)oxide is then heated strongly and reacts as follows: sno2.xh2o(s)sno2(s)+ xh2o(g)the sno2is finally cooled and weighed in the crucible. explain the effect on the calculated atomic weight of tin that would result from each of the following experimental errors: (a)considerable spattering occurs when the nitric acid is added to the tin.(b)the hydrated tin(iv)oxide is not heated sufficiently to change it completely to tin oxide.
Answers: 2
Do you know the correct answer?
The density of lithium metal is 535 kg/m3. what is this density in g/cm3? a) 0.000535 g/cm^3 b) 0.5...
Questions in other subjects:



Mathematics, 05.05.2020 04:04


History, 05.05.2020 04:04

Mathematics, 05.05.2020 04:04


Mathematics, 05.05.2020 04:04


Mathematics, 05.05.2020 04:04