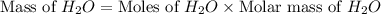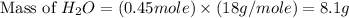Chemistry, 12.08.2019 23:30, nmartin5185
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
ch4 (g) + 2 o2 (g) --> co2 (g) + 2 h2o (l)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, iloveballet1857
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3



Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
Do you know the correct answer?
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
Questions in other subjects:

History, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00


Mathematics, 22.09.2021 14:00


Physics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00


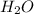 produced will be, 8.1 grams.
produced will be, 8.1 grams.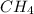 =
= 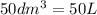
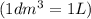
 =
= 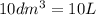
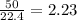 mole of
mole of 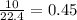 mole of
mole of 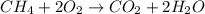
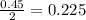 moles of
moles of