
Chemistry, 12.08.2019 22:30, lovecats12
Consider the following redox equation mn(oh)2(s) + mno4 –(aq) mno42 –(aq) (basic solution) when the equation is balanced with smallest whole number coefficients, what is the coefficient for oh –(aq) and on which side of the equation is oh –(aq) present?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
Do you know the correct answer?
Consider the following redox equation mn(oh)2(s) + mno4 –(aq) mno42 –(aq) (basic solution) when th...
Questions in other subjects:

Mathematics, 24.08.2019 17:30

History, 24.08.2019 17:30

Mathematics, 24.08.2019 17:30




Biology, 24.08.2019 17:30

Mathematics, 24.08.2019 17:30


Mathematics, 24.08.2019 17:30



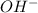 is, 4 and on reactant side of the equation
is, 4 and on reactant side of the equation 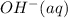 is present.
is present.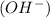 at that side where the less number of hydrogen are present.Now balance the charge.
at that side where the less number of hydrogen are present.Now balance the charge.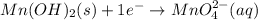 ......(1)
......(1)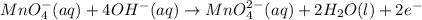 .....(2)
.....(2)



