
Chemistry, 12.08.2019 21:30, rhyanebean6443
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts with oxygen gas, producing carbon dioxide gas and water vapor. calculate the moles of carbon dioxide produced by the reaction of 1.5 mol of oxygen. be sure your answer has a unit symbol, if necessary, and round it to significant digits.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
Do you know the correct answer?
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts...
Questions in other subjects:


Computers and Technology, 25.08.2021 19:30

Chemistry, 25.08.2021 19:30

Mathematics, 25.08.2021 19:30

Mathematics, 25.08.2021 19:30

Mathematics, 25.08.2021 19:30

Business, 25.08.2021 19:30


Mathematics, 25.08.2021 19:30

Mathematics, 25.08.2021 19:30



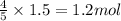 of carbon dioxide.
of carbon dioxide.



