
Chemistry, 12.08.2019 21:20, edfrank6278
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.500 m solution of aspirin has a ph of 1.86. you are interested in learning about the % dissociation in a buffered solution of aspirin, so you make a new 1.00 l solution containing 0.500 moles of aspirin and 0.25 moles of the sodium salt of aspirin. what will the % dissociation be in this new buffered solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, annan65
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3

Chemistry, 21.06.2019 22:30, granthazenp5e9mj
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
Do you know the correct answer?
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.50...
Questions in other subjects:


Computers and Technology, 29.07.2019 07:00




Mathematics, 29.07.2019 07:00



Mathematics, 29.07.2019 07:00


![-log[H^{+}]](/tpl/images/0174/4805/1d5a1.png)
![[H^{+}] = 10^{-pH}](/tpl/images/0174/4805/241df.png)
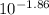
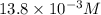
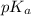 value will be calculated as follows.
value will be calculated as follows.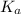 =
= ![\frac{[H^{+}]^{2}}{[Aspirin]}](/tpl/images/0174/4805/0efaa.png)
![\frac{[13.8 \times 10^{-3}]^{2}}{0.50}](/tpl/images/0174/4805/d6d3a.png)
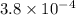
![pK_{a} = -log [K_{a}]](/tpl/images/0174/4805/95c79.png)
![-log [3.8 \times 10^{-4}]](/tpl/images/0174/4805/46d55.png)
![pK_{a} + log \frac{[CH_{3}COO^{-}]}{[Aspirin]}](/tpl/images/0174/4805/cc4ea.png)
![log \frac{[0.25]}{[0.5]}](/tpl/images/0174/4805/52faa.png)
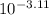
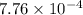 M
M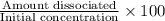
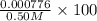
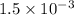 × 100
× 100



