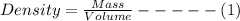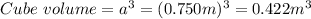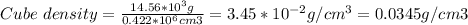Chemistry, 12.08.2019 20:20, kileykittykt8184
Determine the density of the following object in g/cm3. a cube with edge length=0.750 m and mass=14.56 kg. (a) 0.0345 g/cm^3 (b) 1.74 g/cm^3 (c) 670 g/cm^3 (d) 53.8 g/cm^3 (e) 14.6 g/cm^3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1



Chemistry, 23.06.2019 03:30, damyonfenton13
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
Do you know the correct answer?
Determine the density of the following object in g/cm3. a cube with edge length=0.750 m and mass=14....
Questions in other subjects:


History, 13.07.2019 19:50

Mathematics, 13.07.2019 19:50

Mathematics, 13.07.2019 19:50

Social Studies, 13.07.2019 19:50





Mathematics, 13.07.2019 19:50