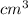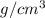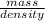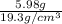Chemistry, 12.08.2019 20:10, Sjanaya853
Given that the density of gold is 19.3 g/cm^3, calculate the volume (in cm^3) of a gold nugget with a mass of 5.98 g. (a) 3.23 cm^3 (b) 5.98 cm^3 (c) 115 cm^3 (d) 0.310 cm^3 (e) 13.3 cm^3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1
Do you know the correct answer?
Given that the density of gold is 19.3 g/cm^3, calculate the volume (in cm^3) of a gold nugget with...
Questions in other subjects:


Health, 12.12.2019 08:31

Chemistry, 12.12.2019 08:31

Mathematics, 12.12.2019 08:31

Mathematics, 12.12.2019 08:31


Biology, 12.12.2019 08:31

History, 12.12.2019 08:31

Chemistry, 12.12.2019 08:31