
Chemistry, 12.08.2019 18:30, briarkaltvedt
Calculate δ h° for the reaction c 4h 4( g) + 2h 2( g) → c 4h 8( g), using the following data: δ h° combustion for c 4h 4( g) = –2341 kj/mol δ h° combustion for h 2( g) = –286 kj/mol δ h° combustion for c 4h 8( g) = –2755 kj/mol

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 10:00, 2019reynolds
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
Do you know the correct answer?
Calculate δ h° for the reaction c 4h 4( g) + 2h 2( g) → c 4h 8( g), using the following data: δ h°...
Questions in other subjects:






History, 09.07.2019 06:30

History, 09.07.2019 06:30

History, 09.07.2019 06:30



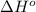
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_{(product)}]-\sum [n\times \Delta H^o_{(reactant)}]](/tpl/images/0174/3643/6872e.png)
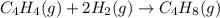
![\Delta H^o_{rxn}=[(1\times \Delta H^o_{(C_4H_8(g))})]-[(1\times \Delta H^o_{(C_4H_4(g))})+(2\times \Delta H^o_{(H_2(g))})]](/tpl/images/0174/3643/605f9.png)
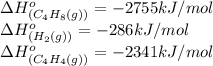
![\Delta H^o_{rxn}=[(1\times (-2755))]-[(1\times (-286))+(2\times (-2341))]\\\\\Delta H^o_{rxn}=2213kJ](/tpl/images/0174/3643/044fa.png)





