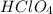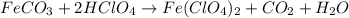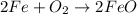Chemistry, 10.08.2019 06:10, briansalazar17
Predict the products of each of the following reactions and then balance the chemical equations.
(a) fe is heated in an atmosphere of steam.
(b) naoh is added to a solution of fe(no3)3.
(c) feso4 is added to an acidic solution of kmno4.
(d) fe is added to a dilute solution of h2so4.
(e) a solution of fe(no3)2 and hno3 is allowed to stand in air.
(f) feco3 is added to a solution of hclo4.
(g) fe is heated in air.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 04:00, BaileyElizabethRay
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
Do you know the correct answer?
Predict the products of each of the following reactions and then balance the chemical equations.
Questions in other subjects:













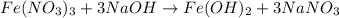
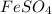 is added to an acidic solution of
is added to an acidic solution of 
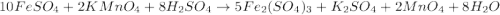


 and
and 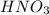 is allowed to stand in air.
is allowed to stand in air.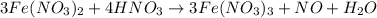
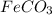 is added to a solution of
is added to a solution of