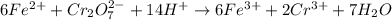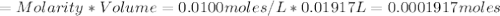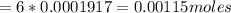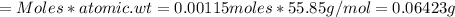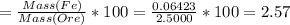Iron(ii) can be oxidized to iron(iii) by dichromate ion, which is reduced to chromium(iii) in acid solution. a 2.5000-g sample of iron ore is dissolved and the iron converted into iron(ii). exactly 19.17 ml of 0.0100 m na2cr2o7 is required in the titration. what percentage of the ore sample was iron?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kingteron5870
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 11:40, heyheyheyhey3
Which of the following is true for a reliable scientific source? it cites logic. it cites opinions. it cites valid data. it cites common sense.
Answers: 2

Chemistry, 23.06.2019 18:00, landofliam30
An engineer designing a new type of engine needs a liquid that can be heated and cooled quickly with as little exchange of energy as possible. which property should the engineer primarily look for in the liquid? a. low thermal conductivity b. low specific heat capacity c. high reactivity d. high internal energy e. high density
Answers: 1
Do you know the correct answer?
Iron(ii) can be oxidized to iron(iii) by dichromate ion, which is reduced to chromium(iii) in acid s...
Questions in other subjects:



Biology, 23.07.2019 16:40

Business, 23.07.2019 16:40

Social Studies, 23.07.2019 16:40

Business, 23.07.2019 16:40

Chemistry, 23.07.2019 16:40


Business, 23.07.2019 16:40

Social Studies, 23.07.2019 16:40