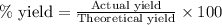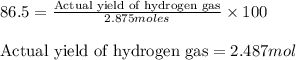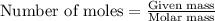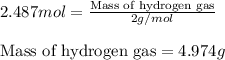Chemistry, 10.08.2019 03:20, jazprincezz7606
If the reaction of 5.75 moles of sodium with excess hydrofluoric acid produced an 86.5% yield of hydrogen gas, what was the actual yield of hydrogen gas?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 08:30, vanessadaniellet21
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 22.06.2019 10:50, mi364
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
Do you know the correct answer?
If the reaction of 5.75 moles of sodium with excess hydrofluoric acid produced an 86.5% yield of hyd...
Questions in other subjects:

World Languages, 15.03.2020 00:44

Computers and Technology, 15.03.2020 00:44




History, 15.03.2020 00:48

Mathematics, 15.03.2020 00:48

Mathematics, 15.03.2020 00:48



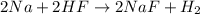
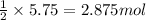 of hydrogen gas.
of hydrogen gas.