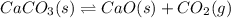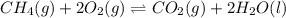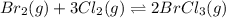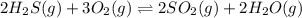Chemistry, 10.08.2019 00:10, dheydar3506
Each of the following reactions is allowed to reach equilibrium in a sealed container. for which of the reactions could you shift the equilibrium to the right by decreasing the pressure?
ch4(g) + 2o2(g) double arrow yields co2(g) + 2h2o(l)
caco3(s) double arrow yields cao(s) + co2(g)
br2(g) + 3cl2(g) double arrow yields 2brcl3(g)
2h2s(g) + 3o2(g) double arrow yields 2so2(g) + 2h2o(g)

Answers: 2
Similar questions

Chemistry, 16.07.2019 06:30, mya1318
Answers: 1

Chemistry, 06.09.2019 04:20, andydiaz1227
Answers: 3

Chemistry, 05.10.2019 04:10, dareaalcaam111
Answers: 2

Chemistry, 01.11.2019 05:31, cynayapartlow88
Answers: 3
Do you know the correct answer?
Each of the following reactions is allowed to reach equilibrium in a sealed container. for which of...
Questions in other subjects:


English, 28.11.2021 21:00



Mathematics, 28.11.2021 21:00




Mathematics, 28.11.2021 21:00

Engineering, 28.11.2021 21:00