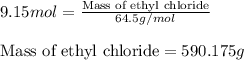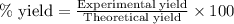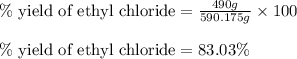Chemistry, 09.08.2019 23:20, 4804397217
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calculate the percent yield of c2h5cl if the reaction of 300 g of ethane with 650 g of chlorine produced 490 g of c2h5cl .

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, barry14201
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 09:30, chy2313
Organisms that live in the alpine and taiga biomes have developed unique adaptations that aid in their survival. moss campion is one of the plants found in the alpine biome. it has small leaves and a cushion shape that protect it from the wind and freezing temperatures in the alpine. how has the moss campion adapted to enable its survival in the alpine biome? a. waxy needles b. cone-shaped c. thin trunks d. low-growing
Answers: 1
Do you know the correct answer?
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calcu...
Questions in other subjects:

Mathematics, 16.12.2020 01:30


Mathematics, 16.12.2020 01:30

Mathematics, 16.12.2020 01:30


Mathematics, 16.12.2020 01:30





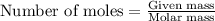 ....(1)
....(1)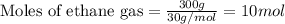
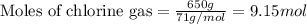
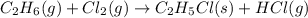
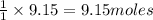 of ethane gas.
of ethane gas.