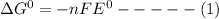Consider the following half reaction in which nitrate is reduced to nitrite: no3– 2h 2e– → no2– h2o. the reaction has a δg°' value of –81 kj/mol. the negative value of δg°' results from the very e°' value of the redox couple no3–/no2–, and it reflects the strong tendency of nitrate to electrons. choose one: a. positive / donateb. negative/ acceptc. negative / donated. positive / accept

Answers: 3
Similar questions

Chemistry, 01.07.2019 11:30, DiamondW1258
Answers: 1

Chemistry, 23.10.2019 04:00, netflixacc0107
Answers: 3

Chemistry, 28.10.2019 21:31, tylorroundy
Answers: 1
Do you know the correct answer?
Consider the following half reaction in which nitrate is reduced to nitrite: no3– 2h 2e– → no2– h2o...
Questions in other subjects:






Mathematics, 20.11.2019 20:31

Mathematics, 20.11.2019 20:31

Mathematics, 20.11.2019 20:31


Chemistry, 20.11.2019 21:31