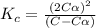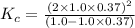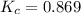Chemistry, 09.08.2019 19:20, pwolfiimp4
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibrium described by the equation n2o4(g) ↔ 2no2(g) if at equilibrium the n2o4 is 37% dissociated, what is the value of the equilibrium constant, kc, for the reaction under these conditions?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, jescanarias22
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 07:30, danielahumajova6
How do you interpret a chromagram for what mixtures contain?
Answers: 1
Do you know the correct answer?
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibrium descr...
Questions in other subjects:

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50




History, 18.03.2021 02:50

Chemistry, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50


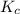 for the reaction is, 0.869
for the reaction is, 0.869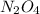 .
.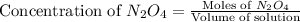
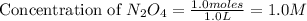
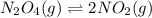
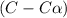
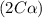
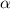 = 37 % = 0.37
= 37 % = 0.37![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0173/7935/271f5.png)