Chemistry, 09.08.2019 17:20, jgnjanjdknsjrr9358
Given the concentrations, calculate the equilibrium constant for this reaction: pcl3(g) +cl2(g)⇌pcl5(g) at equilibrium, the molar concentrations for reactants and products are found to be [pcl3]=0.20 m, [cl2]=0.25 m, and [pcl5]=1.20 m. what is the equilibrium constant (kc) for this reaction? express your answer using two significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, Slycooper5959
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 22.06.2019 20:30, lexibyrd120
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
Do you know the correct answer?
Given the concentrations, calculate the equilibrium constant for this reaction: pcl3(g) +cl2(g)⇌pcl...
Questions in other subjects:


Mathematics, 18.12.2020 19:10

Business, 18.12.2020 19:10






Mathematics, 18.12.2020 19:10



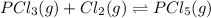
![K_c=\frac{[PCl_5]}{[PCl_3][Cl_2]}](/tpl/images/0173/7310/4c8d0.png)