
Chemistry, 09.08.2019 04:10, alexandrarosete7
Asolution is prepared in which 0.00100 mol ni(no3)2 and 0.500 mol nh3 are dissolved in a total volume of 1.00 l. what is the concentration of ni(h2o)6 21 ions in the solution at equilibrium?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 23.06.2019 07:20, prettydoll19
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3

Chemistry, 23.06.2019 08:50, carlinryan
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2
Do you know the correct answer?
Asolution is prepared in which 0.00100 mol ni(no3)2 and 0.500 mol nh3 are dissolved in a total volum...
Questions in other subjects:

History, 03.12.2020 22:00

Mathematics, 03.12.2020 22:00

History, 03.12.2020 22:00

Spanish, 03.12.2020 22:00

Mathematics, 03.12.2020 22:00





History, 03.12.2020 22:00


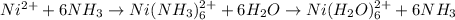
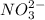 are the separator ions. Hence, molarity of
are the separator ions. Hence, molarity of 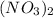 will be calculated as follows.
will be calculated as follows.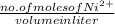
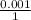 = 0.001 M
= 0.001 M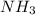 =
= 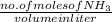
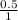 = 0.5 M
= 0.5 M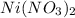 and
and  .
.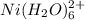 is 1:1.
is 1:1. }{/text{volume in liter}}" alt="Ni(H_{2}O)^{2+}_{6}}" />}{/text{volume in liter}}" />
}{/text{volume in liter}}" alt="Ni(H_{2}O)^{2+}_{6}}" />}{/text{volume in liter}}" />



