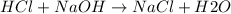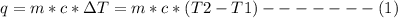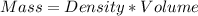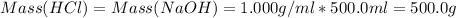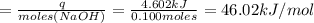Chemistry, 09.08.2019 04:10, Hamadsaqer9
Avolume of 500.0 ml of 0.220 m hcl(aq) was added to a high quality constant-pressure calorimeter containing 500.0 ml of 0.200 m naoh(aq). both solutions have a density of 1.000 g ml-1 and a specific heat of 4.184 j g‑1 oc-1. the temperature of the entire system rose from 25.60 °c to 26.70 °c. calculate the heat of reaction, in kj, per mole of naoh(aq).

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1
Do you know the correct answer?
Avolume of 500.0 ml of 0.220 m hcl(aq) was added to a high quality constant-pressure calorimeter con...
Questions in other subjects:

Mathematics, 17.11.2020 19:40

Mathematics, 17.11.2020 19:40

Mathematics, 17.11.2020 19:40




Business, 17.11.2020 19:40

Mathematics, 17.11.2020 19:40

Engineering, 17.11.2020 19:40