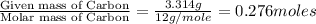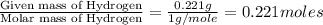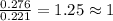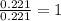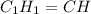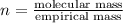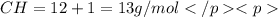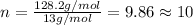Chemistry, 08.08.2019 06:20, alwaysneedhelp84
when 3.539 grams of a hydrocarbon, cxhy, were burned in a combustion analysis apparatus, 12.15 grams of co2 and 1.990 grams of h2o were produced.
in a separate experiment, the molar mass of the compound was found to be 128.2 g/mol. determine the empirical formula and the molecular formula of the hydrocarbon.
enter the elements in the order presented in the question.
empirical formula =
molecular formula =

Answers: 1
Similar questions

Chemistry, 25.08.2019 07:10, ninjaben
Answers: 1

Chemistry, 28.08.2019 16:30, angelica3752
Answers: 1

Chemistry, 31.10.2019 20:31, genesis6154
Answers: 1
Do you know the correct answer?
when 3.539 grams of a hydrocarbon, cxhy, were burned in a combustion analysis apparatus, 12.15 grams...
Questions in other subjects:

Mathematics, 10.10.2019 02:30


Chemistry, 10.10.2019 02:30

Mathematics, 10.10.2019 02:30

English, 10.10.2019 02:30

Mathematics, 10.10.2019 02:30



Mathematics, 10.10.2019 02:30

Mathematics, 10.10.2019 02:30


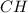 and
and 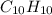 respectively.
respectively.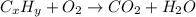
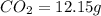
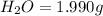
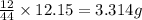 of carbon will be contained.
of carbon will be contained.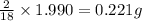 of hydrogen will be contained.
of hydrogen will be contained.