
A2.832 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis and 6.439 grams of co2 and 2.636 grams of h2o are produced. in a separate experiment, the molar mass is found to be 116.2 g/mol. determine the empirical formula and the molecular formula of the organic compound. enter the elements in the order c, h, o empirical formula = molecular formula =

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, pressure772
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 23.06.2019 00:20, cmflores3245
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
Do you know the correct answer?
A2.832 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis a...
Questions in other subjects:

Mathematics, 01.04.2020 01:49

Mathematics, 01.04.2020 01:49

Mathematics, 01.04.2020 01:49

History, 01.04.2020 01:49


English, 01.04.2020 01:49

Mathematics, 01.04.2020 01:49

History, 01.04.2020 01:49



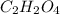 and
and 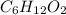 respectively.
respectively.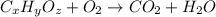
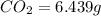
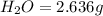
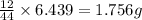 of carbon will be contained.
of carbon will be contained.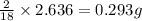 of hydrogen will be contained.
of hydrogen will be contained.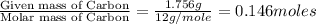
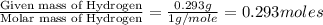
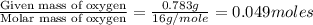
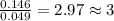
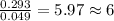
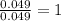
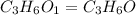
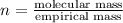
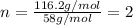
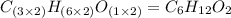
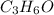 and
and 



