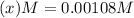Chemistry, 08.08.2019 06:20, baeethtsadia
At a particular temperature, k 5.0 x 10-6 for the reaction 2co2(9) 2co(9) +02(9) if 3.0 moles of co2 is initially placed into a 5.0-l vessel, calculate the equilibrium concentrations of all species. co2] co] o2l [02]-

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, Aminton737
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
Do you know the correct answer?
At a particular temperature, k 5.0 x 10-6 for the reaction 2co2(9) 2co(9) +02(9) if 3.0 moles of co2...
Questions in other subjects:



Mathematics, 23.09.2019 19:30


History, 23.09.2019 19:30

History, 23.09.2019 19:30




Chemistry, 23.09.2019 19:30


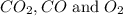 at equilibrium will be, 0.598 M, 0.00216 M and 0.00108 M respectively.
at equilibrium will be, 0.598 M, 0.00216 M and 0.00108 M respectively.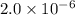
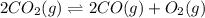
![K_c=\frac{[CO]^2[O_2]}{[CO_2]^2}](/tpl/images/0173/1740/96a03.png)
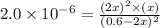
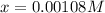
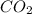 at equilibrium =
at equilibrium = ![(0.6-2x)M=[0.6-2(0.00108)]M=0.598M](/tpl/images/0173/1740/7a4da.png)
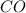 at equilibrium =
at equilibrium = ![(2x)M=[2(0.00108)]M=0.00216M](/tpl/images/0173/1740/eff4f.png)
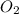 at equilibrium =
at equilibrium =