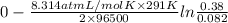Aconcentration cell is built based on the following half reactions by using two pieces of zinc as electrodes, two zn2+ solutions, 0.129 m and 0.427 m, and all other materials needed for a galvanic cell. what will the potential of this cell be when the cathode concentration of zn2+ has changed by 0.047 m at 291 k?
zn2+ + 2 e- ? zn eo = -0.761 v

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3


Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
Do you know the correct answer?
Aconcentration cell is built based on the following half reactions by using two pieces of zinc as el...
Questions in other subjects:


English, 30.07.2019 03:30

Health, 30.07.2019 03:30

Arts, 30.07.2019 03:30



Mathematics, 30.07.2019 03:30

Biology, 30.07.2019 03:30


Mathematics, 30.07.2019 03:30


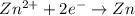 ,
, 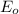 = -0.761 V
= -0.761 V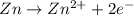 ,
, 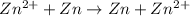
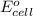 for the given reaction is zero.
for the given reaction is zero.![E^{o}_{cell} - \frac{RT}{nF} ln \frac{[Zn^{2+}]_{products}}{[Zn^{2+}]_{reactants}}](/tpl/images/0173/1699/4d9c9.png)