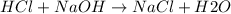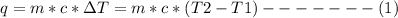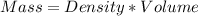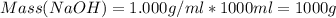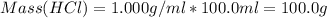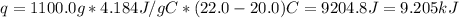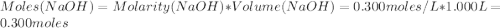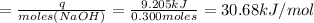Chemistry, 08.08.2019 06:10, joshuahinton45
A1000 ml sample of 0.300 m naoh is mixed with 100.0 ml of 0.300 m hcl in a coffee cup calorimeter. if both solutions are at 20.0 °c and the final temperature of the mixture was 22.0 °c find the heat of neutralization, ahreu in kj/mole. assume no heat is lost to the surroundings, the density of all solutions is 1.00 g/ml, and c, of the mixture is 4.184 j/g·°c

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
Do you know the correct answer?
A1000 ml sample of 0.300 m naoh is mixed with 100.0 ml of 0.300 m hcl in a coffee cup calorimeter. i...
Questions in other subjects:

Mathematics, 23.09.2019 23:20

Biology, 23.09.2019 23:20

Biology, 23.09.2019 23:20

Mathematics, 23.09.2019 23:20

Mathematics, 23.09.2019 23:20




History, 23.09.2019 23:20

Chemistry, 23.09.2019 23:20