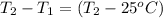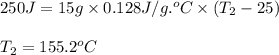Chemistry, 08.08.2019 05:30, jhashknkughb6759
A15.0 g metal sample at 25.0 °c has 250 j of heat added to it. the specific heat of the metal is 0.128 j/g.°c. what is the final temperature?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 12:30, okasiafolk27
15) a substance used in manufacturing gasoline consists of finely divided platinum supported on an inert solid. suppose that the platinum is formed by the high temperature reaction between platinum (iv) oxide and hydrogen gas. the other product is water. a) write and balance the equation b) how many grams of hydrogen are needed to produce 1.0 g of platinum metal? c) how many moles of water are produced at the same time? how many grams? ( show work, .)
Answers: 1

Chemistry, 23.06.2019 13:20, brainbean
Use the periodic table to answer the following questions. what is the predicted order of first ionization energies from highest to lowest for beryllium, calcium, magnesium, and strontium? o be > ca > mg > sr o be > mg > ca > sr o ca > sr> be > mg o sr > ca > mg > be done
Answers: 1
Do you know the correct answer?
A15.0 g metal sample at 25.0 °c has 250 j of heat added to it. the specific heat of the metal is 0.1...
Questions in other subjects:




Mathematics, 07.12.2019 06:31


Mathematics, 07.12.2019 06:31

Chemistry, 07.12.2019 06:31


Arts, 07.12.2019 06:31

History, 07.12.2019 06:31


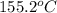
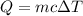
 = change in temperature =
= change in temperature =