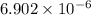Answers: 3
Similar questions

Chemistry, 06.07.2019 11:30, astorkid
Answers: 1

Chemistry, 09.07.2019 03:10, chubbyxturtle
Answers: 3
Do you know the correct answer?
A2.35l solution contains 2.20 mol of weak acid hx. if 1.15 mol naoh is added to this solution, the p...
Questions in other subjects:



Social Studies, 31.08.2019 19:30








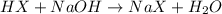
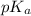 will be as follows.
will be as follows.![pK_{a} + \frac{log[NaX]}{[HX]}](/tpl/images/0173/1558/694ef.png)
![pK_{a} = pH - log \frac{log[NaX]}{[HX]}](/tpl/images/0173/1558/95a26.png)
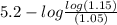
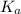 =
=