
Chemistry, 08.08.2019 00:20, aroland1990x
Calculate the δ h°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide. δ h°f [caco3(s)] = –1206.9 kj/mol; δ h°f [cao(s)] = –635.1 kj/mol; δ h°f [co2(g)] = –393.5 kj/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 21.06.2019 22:30, Cnolteb5663
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b. slope c. benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 04:00, kichensides
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
Do you know the correct answer?
Calculate the δ h°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide...
Questions in other subjects:





Geography, 03.04.2020 16:18







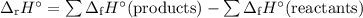
![\begin{array}{rcl}\Delta_{\text{r}}H^{\circ}& = & [(-635.1 + (-393.5)] - (-1206.9)\\& = & -1028.6 +1206.9\\& = & \mathbf{178.3}\\\end{array}\\\text{The enthalpy of decomposition is } \boxed{\textbf{178.3 kJ/mol}}](/tpl/images/0172/9753/c40b5.png) l}}
l}}



