
Chemistry, 07.08.2019 03:10, Jazminnexoxo1093
The following thermodynamic data are available for octane, oxygen gas, carbon dioxide gas, water, and water vapor: molecule δh∘f (kj/mol) c8h18(l) −250.1 o2(g) 0 co2(g) −393.5 h2o(l) −285.8 h2o(g) −241.8 part b calculate δhrxn for the combustion of octane by using enthalpies of formation from the transition above. express the energy in kilojoules per mole to three significant figures. δhrxn δ h r x n = nothing kj/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, nadinealonzo6121
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 21.06.2019 20:30, mimithurmond03
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
Do you know the correct answer?
The following thermodynamic data are available for octane, oxygen gas, carbon dioxide gas, water, an...
Questions in other subjects:

Mathematics, 26.05.2021 18:40

History, 26.05.2021 18:40



Mathematics, 26.05.2021 18:40

Mathematics, 26.05.2021 18:40


Mathematics, 26.05.2021 18:40



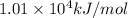
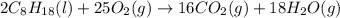
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0172/5689/45485.png)
![\Delta H^o_{rxn}=[(16\times \Delta H^o_f_{(CO_2(g))})+(18\times \Delta H^o_f_{(H_2O(g))})]-[(2\times \Delta H^o_f_{(C_8H_{18}(l))})+(25\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0172/5689/36b5a.png)
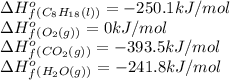
![\Delta H^o_{rxn}=[(16\times (-393.5))+(18\times (-241.8))]-[(2\times (-250.1))+(25\times (0))]=10148.2kJ/mol=1.01\times 10^4kJ/mol](/tpl/images/0172/5689/a24a9.png)




