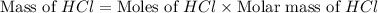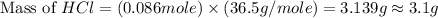Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30.0 g of hcl (fw = 36.5 g/mol) react according to the following chemical equation? mno2 + 4 hcl ® mncl2 + cl2 + 2 h2o3.1 g hcl23.3 g hcl4.02 g mno28.0 g mno212.1 g mno2

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1


Chemistry, 22.06.2019 19:10, aamu15
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
Do you know the correct answer?
Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30...
Questions in other subjects:







Mathematics, 14.10.2019 20:20




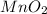 = 16.0 g
= 16.0 g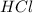 = 30.0 g
= 30.0 g
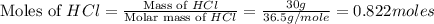
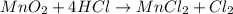
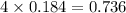 moles of
moles of