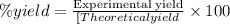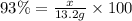Chemistry, 06.08.2019 05:10, cadanceowasso
When octane (c8h18) is burned in a particular internal combustion engine, the yield of carbon dioxide is 93%. what mass of carbon dioxide will be produced in this engine when 15.0 g of octane (mw = 114.0 g/mol) is burned with 15.0 g of oxygen gas (mw = 32.0 g/mol)? 2c8h18 + 25 o2 --> 16 co2 + 18 h2o
a.12g b.13g c.21g d.43g e.54g

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, ayoismeisjjjjuan
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x. xx ml
Answers: 1


Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
Do you know the correct answer?
When octane (c8h18) is burned in a particular internal combustion engine, the yield of carbon dioxid...
Questions in other subjects:

Mathematics, 08.11.2020 14:00


English, 08.11.2020 14:00



English, 08.11.2020 14:00

Social Studies, 08.11.2020 14:00




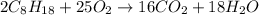
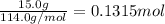
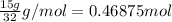
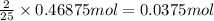 of octane
of octane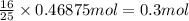 of carbon-dioxide
of carbon-dioxide